Aclerastide
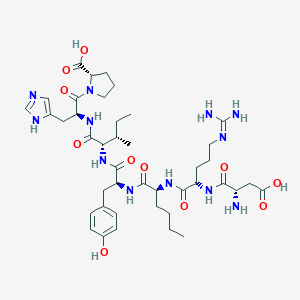
Aclerastide is being investigated for the treatment of diabetic wound healing, and more specifically for diabetic foot ulcers (DFU). It failed Phase III clinical trials in November 2015 for treatment of DFU, where it was applied daily for several weeks. Aclerastide, also known as DSC-127, and NorLeu3-A(1-7), is a potent peptide analog of angiotensin II, a growth factor that plays an important role in dermal repair and wound healing by promoting angiogenesis, producing reactive oxygen species, and inducing migration of fibroblasts and keratinocytes. In animal models, aclerastide showed more rapid deposition of collagen, acceleration of epithelial growth, altered leukocyte infiltration, and increased blood vessel formation.
Why do you need a Aclerastide monograph?
Superior Toxicology and Wellness’s monographs are a cost-effective and convenient way to meet the requirements for PDEs (ADEs)
- The numerical OEL and control band assignment needed to determine the level of required engineering controls and personal protective equipment
- A listing of all cited references utilized in the derivation of the OEL and ADE
- An expert review and discussion with respect to the critical endpoints of concern, the rationale for the choice of endpoints, and dose that is to be used in the derivation of the ADE (PDE), as required by the EMA’s Guideline on setting health-based exposure limits for use in risk identification in the manufacture of different medicinal products in shared facilities
Benefits of Superior Toxicology and Wellness monographs:
- Full drug documentation. The report consists of 10-12 pages in length, with calculations and cited references.
- Quick delivery! 24-48 hours of purchase. Sometimes even an instant download.
- Save money. Similar reports will cost you potentially 5-12 times more.
- Search our other reports!


Reviews
There are no reviews yet.